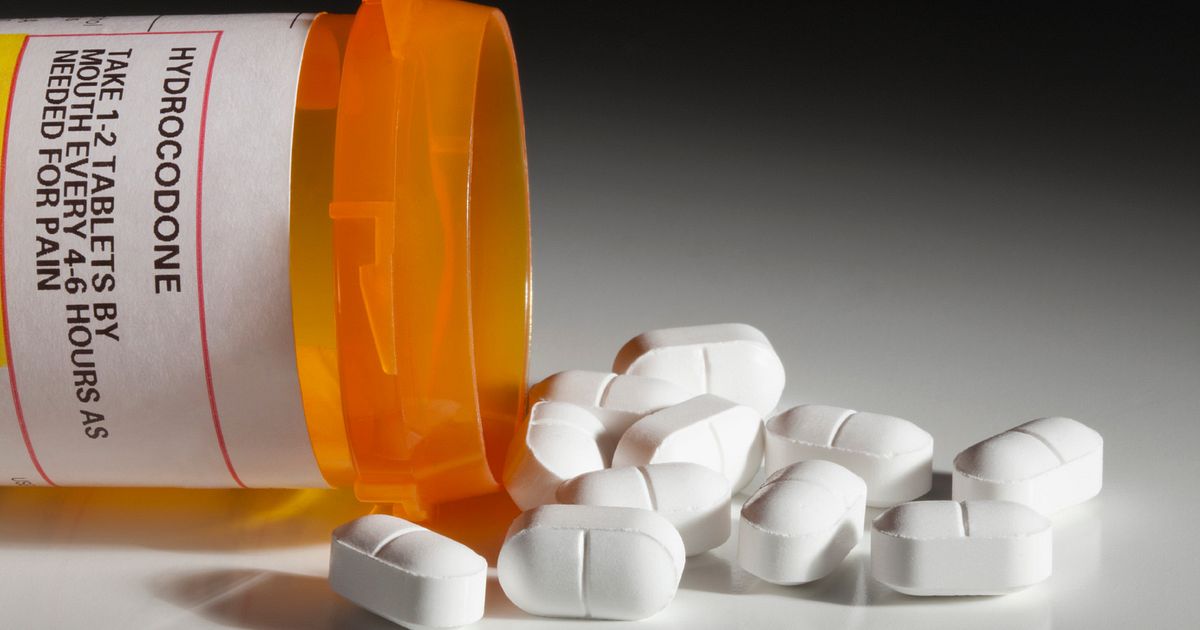
The FDA Memo states that Opioid Pain Medicines should NOT be used for an extended period!
Drug Safety Communication: All Opioid Pain Medicines – FDA Updates Prescribing Information to Provide Additional Guidance for Safe Use
These Are The New OPIOID PAIN MEDICATION Guidelines By The FDA …
Issued on April 13, 2023 by The FDA, reported by Drugs.com:
[playht_listen_button inline=”yes” tag=”p”]
The FDA is requiring several updates to the prescribing information for both immediate-release (IR) and extended release/long acting (ER/LA) opioid pain medicines. This includes stating for all opioid pain that the risk of overdose increases as the dose increases.
- The updates to IR opioids state these products should not be used for an extended period unless the pain remains severe enough to require them and alternative treatments continue to be inadequate, and that many acute pain conditions treated in the outpatient setting require no more than a few days of an opioid pain medicine. This may include pain occurring with a number of surgical conditions or musculoskeletal injuries.
- The FDA is also updating the approved use for ER/LA opioid pain medicines to recommend they be reserved for severe and persistent pain that requires an extended treatment period with a daily opioid pain medicine and for which alternative treatment options are inadequate.
- The FDA is also adding a new warning about opioid-induced hyperalgesia (OIH) for both IR and ER/LA opioid pain medicines. This includes information describing the symptoms that differentiate OIH from opioid tolerance and withdrawal.
- Information in the Boxed Warning, FDA’s most prominent warning, for all IR and ER/LA opioid pain medicines will be updated and reordered to elevate the importance of warnings concerning life-threatening respiratory depression, and risks associated with using opioid pain medicines in conjunction with benzodiazepines or other medicines that depress the central nervous system (CNS).
- Other changes are also being required to several sections of the prescribing information, including to the Indications and Usage, Dosage and Administration, and Warnings and Precautions sections. The FDA is also requiring updates to the existing patient Medication Guides to help educate patients and caregivers about these risks.
[fyrebox oid=”pNvEGJVD0″ gid=”88EpmjwZA”]